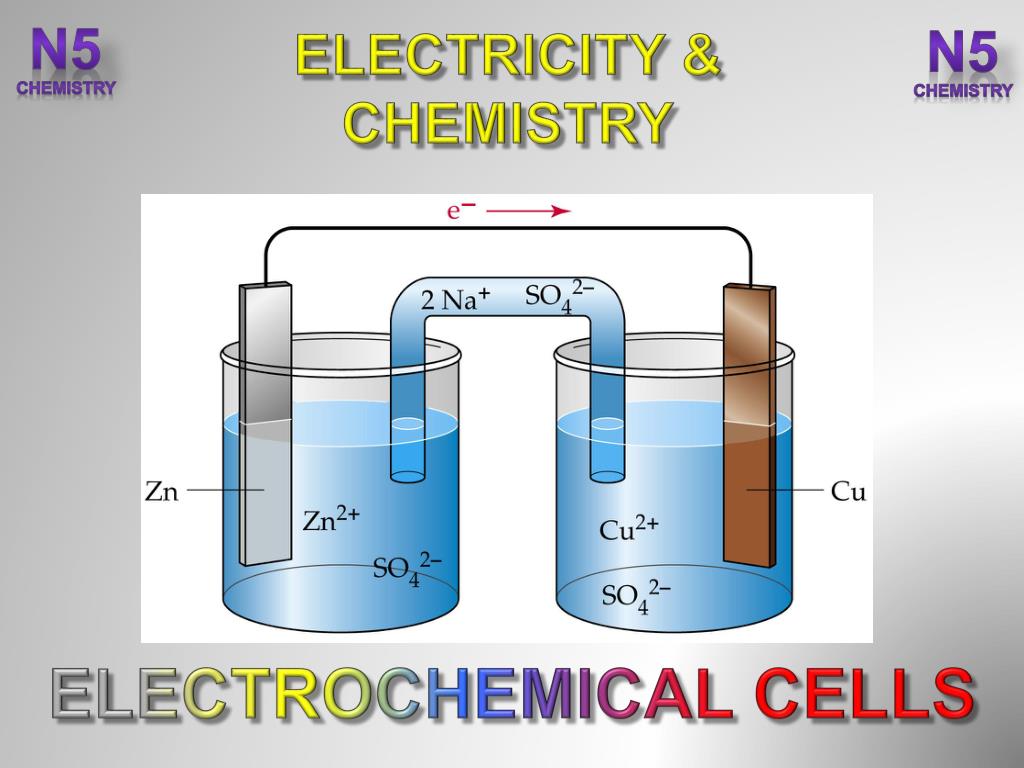
Electrochemistry Cells Definition . Define electrolysis and list several of its applications. List some of the characteristics, applications and limitations of cells and batteries. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an electrochemical cell. Galvanic cells, which are also known as voltaic cells, use chemical reactions to generate electricity. Galvanic cells and electrolytic cells. what is an electrochemical cell? an electrochemical cell is a device that generates a potential difference between electrodes using chemical reactions. An electrochemical cell is a device which either uses chemical reactions occurring in it to. There are two types of electrochemical cells: components of electrochemical cells. Describe the basic components of electrochemical cells. Know the difference between galvanic and electrolytic cells. This kind of cell includes the galvanic,. Galvanic cells and electrolytic cells are examples of electrochemical cells. an electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction.
from www.slideserve.com
Define electrolysis and list several of its applications. an electrochemical cell is a device that generates a potential difference between electrodes using chemical reactions. Galvanic cells, which are also known as voltaic cells, use chemical reactions to generate electricity. An electrochemical cell is a device which either uses chemical reactions occurring in it to. Galvanic cells and electrolytic cells are examples of electrochemical cells. There are two types of electrochemical cells: Galvanic cells and electrolytic cells. Know the difference between galvanic and electrolytic cells. List some of the characteristics, applications and limitations of cells and batteries. Describe the basic components of electrochemical cells.
PPT ELECTROCHEMICAL CELLS PowerPoint Presentation, free download ID
Electrochemistry Cells Definition Know the difference between galvanic and electrolytic cells. An electrochemical cell is a device which either uses chemical reactions occurring in it to. Galvanic cells, which are also known as voltaic cells, use chemical reactions to generate electricity. Describe the basic components of electrochemical cells. components of electrochemical cells. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an electrochemical cell. This kind of cell includes the galvanic,. An electrochemical cell splits the oxidant and reductant in a manner that allows. Know the difference between galvanic and electrolytic cells. List some of the characteristics, applications and limitations of cells and batteries. Galvanic cells and electrolytic cells are examples of electrochemical cells. Define electrolysis and list several of its applications. what is an electrochemical cell? There are two types of electrochemical cells: Galvanic cells and electrolytic cells. an electrochemical cell is a device that generates a potential difference between electrodes using chemical reactions.
From lessonschoolallodial.z13.web.core.windows.net
Electrochemical Cells In Chemistry Electrochemistry Cells Definition Define electrolysis and list several of its applications. List some of the characteristics, applications and limitations of cells and batteries. an electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. Describe the basic components of electrochemical cells. Galvanic cells and electrolytic cells. An electrochemical cell splits the oxidant and reductant. Electrochemistry Cells Definition.
From guidelisteickhoff.z21.web.core.windows.net
Electrochemistry Cell Diagram Electrochemistry Cells Definition an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an electrochemical cell. Galvanic cells and electrolytic cells are examples of electrochemical cells. List some of the characteristics, applications and limitations of cells and batteries. An electrochemical cell is a device which either. Electrochemistry Cells Definition.
From circuittawnilynne2461.z14.web.core.windows.net
Electrochemistry Cell Diagram Electrochemistry Cells Definition Define electrolysis and list several of its applications. There are two types of electrochemical cells: an electrochemical cell is a device that generates a potential difference between electrodes using chemical reactions. Galvanic cells and electrolytic cells. Know the difference between galvanic and electrolytic cells. an apparatus that is used to generate electricity from a spontaneous redox reaction or,. Electrochemistry Cells Definition.
From materiallibrarycody.z19.web.core.windows.net
Diagram Of Electrochemical Cell Electrochemistry Cells Definition Galvanic cells and electrolytic cells are examples of electrochemical cells. There are two types of electrochemical cells: This kind of cell includes the galvanic,. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an electrochemical cell. an electrochemical cell is a. Electrochemistry Cells Definition.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types Electrochemistry Cells Definition List some of the characteristics, applications and limitations of cells and batteries. This kind of cell includes the galvanic,. an electrochemical cell is a device that generates a potential difference between electrodes using chemical reactions. Galvanic cells, which are also known as voltaic cells, use chemical reactions to generate electricity. an electrochemical cell is a device that produces. Electrochemistry Cells Definition.
From mmerevise.co.uk
Electrochemical Cells Worksheets and Revision MME Electrochemistry Cells Definition an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an electrochemical cell. what is an electrochemical cell? Galvanic cells and electrolytic cells are examples of electrochemical cells. There are two types of electrochemical cells: Describe the basic components of electrochemical cells.. Electrochemistry Cells Definition.
From worksheetfullrusskies.z21.web.core.windows.net
Diagram Of Electrochemical Cell Electrochemistry Cells Definition what is an electrochemical cell? Describe the basic components of electrochemical cells. Define electrolysis and list several of its applications. This kind of cell includes the galvanic,. an electrochemical cell is a device that generates a potential difference between electrodes using chemical reactions. An electrochemical cell is a device which either uses chemical reactions occurring in it to.. Electrochemistry Cells Definition.
From 2012books.lardbucket.org
Electrochemistry Electrochemistry Cells Definition Define electrolysis and list several of its applications. An electrochemical cell splits the oxidant and reductant in a manner that allows. Describe the basic components of electrochemical cells. This kind of cell includes the galvanic,. an electrochemical cell is a device that generates a potential difference between electrodes using chemical reactions. Know the difference between galvanic and electrolytic cells.. Electrochemistry Cells Definition.
From mmerevise.co.uk
Electrochemical Cells MME Electrochemistry Cells Definition An electrochemical cell is a device which either uses chemical reactions occurring in it to. There are two types of electrochemical cells: an electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. This kind of cell includes the galvanic,. Describe the basic components of electrochemical cells. Galvanic cells and electrolytic. Electrochemistry Cells Definition.
From mavink.com
Diagram Of Electrochemical Cell Electrochemistry Cells Definition components of electrochemical cells. Describe the basic components of electrochemical cells. Galvanic cells, which are also known as voltaic cells, use chemical reactions to generate electricity. an electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. Know the difference between galvanic and electrolytic cells. An electrochemical cell splits the. Electrochemistry Cells Definition.
From www.youtube.com
electrochemical cell electrochemistry class 12 chemistry subject notes Electrochemistry Cells Definition List some of the characteristics, applications and limitations of cells and batteries. There are two types of electrochemical cells: This kind of cell includes the galvanic,. Galvanic cells and electrolytic cells. An electrochemical cell is a device which either uses chemical reactions occurring in it to. Know the difference between galvanic and electrolytic cells. an apparatus that is used. Electrochemistry Cells Definition.
From www.collegesearch.in
Electrochemical Cell Definitions, Examples, Electrochemistry Electrochemistry Cells Definition Know the difference between galvanic and electrolytic cells. what is an electrochemical cell? an electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. An electrochemical cell splits the oxidant and reductant in a manner that allows. List some of the characteristics, applications and limitations of cells and batteries. This. Electrochemistry Cells Definition.
From www.youtube.com
AP Chemistry Electrochemistry Cell Potentials YouTube Electrochemistry Cells Definition components of electrochemical cells. An electrochemical cell splits the oxidant and reductant in a manner that allows. This kind of cell includes the galvanic,. There are two types of electrochemical cells: Define electrolysis and list several of its applications. an electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction.. Electrochemistry Cells Definition.
From www.slideserve.com
PPT Electrochemical Cells PowerPoint Presentation, free download ID Electrochemistry Cells Definition Galvanic cells, which are also known as voltaic cells, use chemical reactions to generate electricity. Galvanic cells and electrolytic cells. an electrochemical cell is a device that generates a potential difference between electrodes using chemical reactions. An electrochemical cell splits the oxidant and reductant in a manner that allows. This kind of cell includes the galvanic,. An electrochemical cell. Electrochemistry Cells Definition.
From learningschoolletopisaln.z22.web.core.windows.net
Types Of Electrochemical Cells Pdf Electrochemistry Cells Definition components of electrochemical cells. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an electrochemical cell. Galvanic cells, which are also known as voltaic cells, use chemical reactions to generate electricity. List some of the characteristics, applications and limitations of cells. Electrochemistry Cells Definition.
From www.youtube.com
Electrochemistry Classification Type Of Cell Electrochemical Electrochemistry Cells Definition An electrochemical cell is a device which either uses chemical reactions occurring in it to. List some of the characteristics, applications and limitations of cells and batteries. an electrochemical cell is a device that generates a potential difference between electrodes using chemical reactions. what is an electrochemical cell? Define electrolysis and list several of its applications. This kind. Electrochemistry Cells Definition.
From saylordotorg.github.io
Electrochemistry Electrochemistry Cells Definition Galvanic cells, which are also known as voltaic cells, use chemical reactions to generate electricity. Galvanic cells and electrolytic cells are examples of electrochemical cells. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an electrochemical cell. components of electrochemical cells.. Electrochemistry Cells Definition.
From www.pearson.com
Electrochemical Cells Video Tutorial & Practice Channels for Pearson+ Electrochemistry Cells Definition components of electrochemical cells. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an electrochemical cell. List some of the characteristics, applications and limitations of cells and batteries. an electrochemical cell is a device that produces an electric current from. Electrochemistry Cells Definition.